
 Every day we experience a variety of sensations from our inner and outer environment that help shaping our behavior and wellbeing: shafts of sunlight that touch our skin can lift our spirits after a long cold winter, while a cool breeze can refresh us on a hot summer day. Responsible for our ability to detect these varied stimuli are somatosensory neurons that come in diverse subtypes, each specialized to detect and transduce specific sensory modalities. But besides detecting stimuli that are associated with a positive valence, these neurons are also indispensable to protect our integrity by detecting harmful insults, leading to pain that could result in tissue damage, if we do not react in an appropriate manner. Unfortunately, genetic mutations, certain diseases or traumata causing persistent tissue damage can perturb this warning and protecting system and causes pain to become permanent – a status almost every fifth person in the world is suffering from.
Every day we experience a variety of sensations from our inner and outer environment that help shaping our behavior and wellbeing: shafts of sunlight that touch our skin can lift our spirits after a long cold winter, while a cool breeze can refresh us on a hot summer day. Responsible for our ability to detect these varied stimuli are somatosensory neurons that come in diverse subtypes, each specialized to detect and transduce specific sensory modalities. But besides detecting stimuli that are associated with a positive valence, these neurons are also indispensable to protect our integrity by detecting harmful insults, leading to pain that could result in tissue damage, if we do not react in an appropriate manner. Unfortunately, genetic mutations, certain diseases or traumata causing persistent tissue damage can perturb this warning and protecting system and causes pain to become permanent – a status almost every fifth person in the world is suffering from.
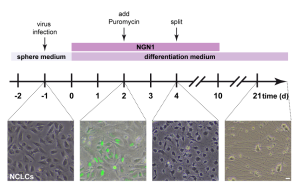
In the Siemens lab, we are interested in recapitulating sensory neuron development in vitro and attempt to generate homogenous populations of specific sensory neuron subtypes from human pluripotent stem cells (hESCS and hIPSCs). To this end, we developed various differentiation strategies based on a two-step process: in a first step we generate neural crest-like cells, the in vivo progenitors of sensory neurons. These cells have the capacity to differentiate further into sensory neurons, either by a growth factor driven approach or by virally induced forced expression of certain transcription factors.
The former results in the generation of large mechanoreceptor-like cells showing molecular and functional characteristics of low threshold mechanoreceptors, a sensory neuron subtype important for detecting touch and other low threshold mechanical stimuli. The latter results in the generation of functional sensory neurons showing characteristics of nociceptor-like cells such as expression of Nav1.8 and responsiveness to the TRPV1 agonist Capsaicin.
These cells serve as a tool to investigate specific aspects of sensory neurobiology in a human cell-based model system.
Our scientific aims are:
(i) Understanding developmental aspects of sensory neuron generation. We believe that this approach will yield new insight into sensory neuron differentiation that has escaped previous genetic studies using complex model organisms.
(ii) Investigating aspects of sensation in nociceptor-like cells and their synaptic transmission under normal and diseased conditions. Thereby we hope to find pathways that help to explain how normal sensitization can turn into chronic states. This project is supported by a collaborative research center funded by the German Research Foundation (SFB1158) in collaboration with Claudio Acuna (group leader at Heidelberg University, funded by the Chica and Heinz Schaller Foundation).